Jianzhong Liu

l ADDRESS:
Address: State Key Laboratory of Natural and Biomimetic Drugs,
Peking University, Xueyuan Road 38, Haidian District
Beijing 100191, P. R. China
Mobile Phone: +86-13501182807
Email: liujianzhong@pku.edu.cn
l PERSONAL PARTICULARS:
Date of Birth: Oct 13, 1988 Sex: Male
Marital Status: Married Health: Good
l EDUCATION:
2015.9-present: Ph. D. Student, Candidate, Organic and Medicinal Chemistry, Peking University. Advisor: Prof. Ning Jiao.
2012.9-2015.6: Master. D., Medicinal Chemistry, Chengdu Institute of Biology, University of Chinese Academy of Sciences. Advisor: Prof. Xiaoxia Lu. Including: 2013.9-2015.3, South China University of Technology. (Co-training student, Director: Prof. Wei Zeng).
2007.9-2011.6: Bachelor. D., Pharmacy, Henan University. Advisor: Prof. Fuli Zhang.
l RESEARCH INTEREST:
n Organometallic Chemistry, C-H/C-C bond functionalization; Radical Chemistry.
n Discovery & development of new reagent and new strategy for nitrogen-atom (NH/NH2 group) installation.
n Pharmaceutical diversification (the synthesis and late-stage modification of bioactive natural products and drugs).
l RESEARCH EXPERIENCE:
2015.9-now: Ph. D., Advisor: Professor Ning Jiao.
Projects including:
1. Primary amination of arenes via C-H bond activation driven by new amination reagent.
2. Anilines synthesis via site-directed carbon-carbon amination of alkylarenes.
3. Novel transformation of styrenes via untouched C(Ar)-C(alkenyl) single bond cleavage.
4. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles
5. Cinnamyl aldehydes synthesis from olefins by selective allylic C-C bond cleavage.
2012.9-2015.6: M. D., Advisor: Professor Xiaoxia Lu; Professor Wei Zeng.
Projects: Directed Arylation of Unactivated Csp3-H Bonds
l HONORS AND AWARDS:
1. Merit Student of University of Chinese Academy of Sciences, 2013.
2. Peking University Innovation Award, 2016.
3. Peking University Excellent Research Award, 2016.
4. National Scholarship (for PhD student, top 2%), 2018.
5. Excellent Graduate student of Peking University, 2018 .
6. Post Award of the 13th International Symposium on Activation of Dioxygen and Homogeneous Catalysis, 2018.
l PUBLICATIONS:
1. Jianzhong Liu, Xiao Luo, Xu Qiu, Cheng Zhang, Jun Pan, Ning Jiao*. Selective C(Ar)-C(Alkenyl) single Bond Amination of Styrenes: Highly Efficient Approach to Primary Anilines and phenols. (to be submited).
2. Jianzhong Liu, Cheng Zhang, Ziyao Zhang, Xiaojin Wen, Xiaodong Dou, Jialiang Wei, Xu Qiu, Song, Song, Ning Jiao*. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles. Science, 2019, 10.1126/science.aay950.
3. Jianzhong Liu, Xu Qiu, Xiaoqiang Huang, Xiao Luo, Cheng Zhang, Jialiang Wei, Jun Pan, Yujie Liang, Yuchao Zhu, Qixue Qin, Song Song, Ning Jiao*. From alkylarenes to anilines via site-directed carbon–carbon amination. Nature Chemistry. 2019, 11, 71.
4. Jianzhong Liu+, Xiaojin Wen+, Chong Qin+, Xinyao Li, Xiao Luo, Ao Sun, Bencong Zhu, Song Song*, Ning Jiao*. Oxygenation of Simple Olefins through Selective Allylic C-C Bond Cleavage: A Direct Approach to Cinnamyl Aldehydes. Angew. Chem. Int. Ed. 2017, 56, 11940.
5. Jianzhong Liu, Kai Wu, Tao Shen, Yujie Liang, Miancheng Zou, Yuchao Zhu, Xinwei Li, Xinyao Li, Ning Jiao*. Fe-Catalyzed Amination of (Hetero)Arenes with a Redox-Active Aminating Reagent under Mild Conditions. Chem.–Eur. J. 2017, 23, 563.
6. Jianzhong Liu, Ying Xie, Wei Zeng*, Dongen Lin*, Yuanfu Deng*, Xiaoxia Lu*. Pd(II)-Catalyzed Pyridine N-Oxides Directed Arylation of Unactivated Csp3−H Bonds. J. Org. Chem.2015, 80, 4618.
7. Yeerlan Adeli, Kaimeng Huang, Yujie Liang, Yangye Jiang, Jianzhong Liu, Song Song, Cheng-Chu Zeng*, Ning Jiao*. Electrochemically Oxidative C-C Bond Cleavage of Alkylarenes for Anilines Synthesis. ACS Catal. 2019, 9, 2063.
8. Jun Pan+, Xinyao Li+, Fengguirong Lin, Jianzhong Liu, Ning Jiao*. Chemoselective Nitrosylation of Anilines and Alkynes via Fragmentary or Complete NO Incorporation. Chem. 2018, 4, 1427.
9. Tao Shen, Bencong Zhu, Fengguirong Lin, Jun Pan, Jialiang Wei, Xiao Luo, Jianzhong Liu, Ning Jiao*. Direct Synthesis of Structurally Divergent Indole Alkaloids from Simple Chemicals. Chin. J. Chem. 2018, 36, 815.
10. Xiaoyang Wang, Xu Qiu, Jialiang Wei, Jianzhong Liu, Song Song, Wen Wang*, Ning Jiao*.Cu-Catalyzed Aerobic Oxidative Sulfuration/Annulation Approach to Thiazoles via Multiple Csp3-H Bond Cleavage. Org. Lett. 2018, 20, 2632.
11. Song Song, Yiqun Zhang, Adeli, Yeerlan, Bencong Zhu, Jianzhong Liu, Ning Jiao* Cs2CO3-Catalyzed Aerobic Oxidative Cross-Dehydrogenative Coupling of Thiols with Phosphonates and Arenes. Angew. Chem. Int. Ed. 2017, 56, 2487.
12. Miancheng Zou, Jianzhong Liu, Conghui Tang, Ning Jiao*. Rh-Catalyzed N-O Bond Cleavage of Anthranil: A C-H Amination Reagent for Simultaneous Incorporation of Amine and a Functional Group. Org. Lett. 2016, 18, 3030.
13. Conghui Tang, Miancheng Zou, Jianzhong Liu, Xiaojin Wen, Xiang Sun, Yiqun Zhang, Ning Jiao*. Rh-Catalyzed Direct Amination of Unactivated C(sp3)-H bond with Anthranils Under Mild Conditions. Chem. Eur. J. 2016, 22, 11165.
14. Yu-Feng Liang, Xiaoyang Wang, Conghui Tang, Tao Shen, Jianzhong Liu, Ning Jiao*. NHPI and palladium co-catalyzed aerobic oxidative acylation of arenes through a radical process. Chem. Commun. 2014, 52, 1416.
l PATENTS:
Ning Jiao, Jianzhong Liu, Song Song. An efficient synthetic method for anilines and its derivatives. Chinese Patent, application number: 201811064304.9, application date 12.09.2018.
Ning Jiao, Jianzhong Liu, Cheng Zhang. The application of highly efficient nitrogenantion reagent. Chinese Patent, application number: 201910943969.5, application date 09.30.2019.
l ACADEMIC ACTIVITIES:
1. The 8th National Organic Chemistry Conference, poster presentation, Chongqing, China. Dec. 2013.
2. The 10th National Organic Chemistry Conference, poster presentation, Shenzhen, China. Dec. 2017
3. The 13th International Symposium on Activation of Dioxygen and Homogeneous Catalysis, poster presentation, Xi’an, China. June. 2018.
For research summary, please see below in the next page:
Research Summary
Jianzhong Liu (Peking University)
Supervisor: Prof. Ning Jiao
During my Ph. D studies, my research mainly focused on the discovery and development of new reagent and new reaction for the high-quality utilization of bulk chemicals and pharmaceutical diversification through the highly efficient nitrogenation and oxygenation reactions, aiming to develop some green and efficient synthetic methodologies for the synthesis of N/O-containing compounds via C-H and/or C-C bond cleavage. Especially, we have developed three novel synthetic routes to the primary anilines, contributing practical site-specific pathway for substituted anilines synthesis. My M. S. studies mainly focused on palladium catalyzed arylation of unactivated Csp3-H Bonds via bidentate auxiliary-directed strategy. Details of my research are shown below:
1. Primary Amination of (Hetero)Arenes with a Redox-Active Aminating Reagent under Mild Conditions.
Primary amines are among the most important chemicals and have been
widely applied in the synthesis of natural products, pharmaceuticals,
agrochemicals, dyes, and polymers. Traditional approaches to anilines generally
rely on the prefunctionalized substrates. Thus, with respect to atom- and step-
economic synthetic methodology, direct C-H amination of arenes has been on the
cutting edge in organic chemistry. However, this strategy often focuses on the
formation of other types of aminated products. The construction of primary
anilines through C-H bond activation is still challenging and has rarely been
reported because 1) the amine precursors and the primary aniline products are usually
unstable under the strong oxidative conditions required in most C-H activation
reactions; 2) the aniline products may chelate to the metal center and poison
the transition metals; 3) compared with other amination reagent, the reactive
activity of NH2-source is lower due to the unfavorable electronic
effect. Herein a novel and efficient Fe-catalyzed direct C-H amination (NH2)
of arenes is developed using a new redox-active aminating reagent. The reaction
is simple, and can be performed under air, mild, and redox-neutral conditions,
and thereby enabling the late-stage modification of bioactive compounds.
Mechanistic studies demonstrate that a radical pathway could be involved in
this transformation.
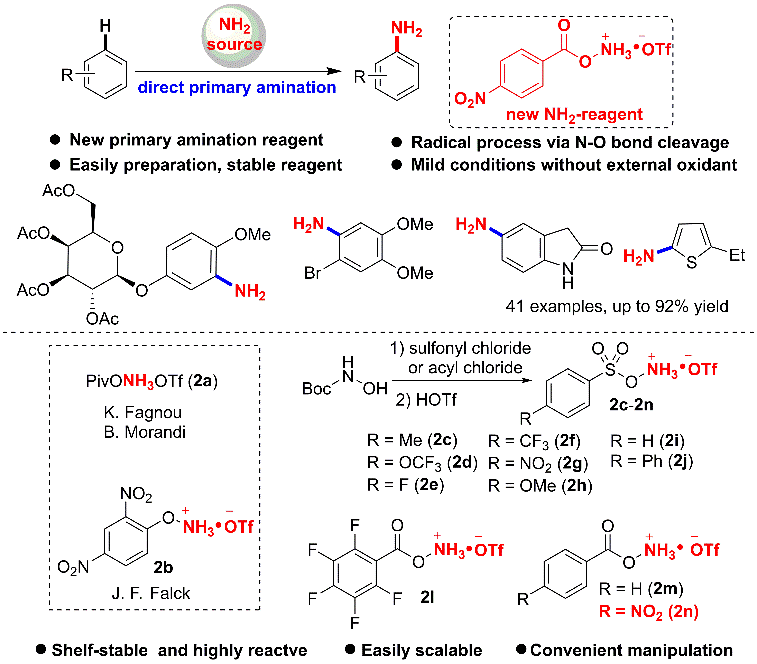
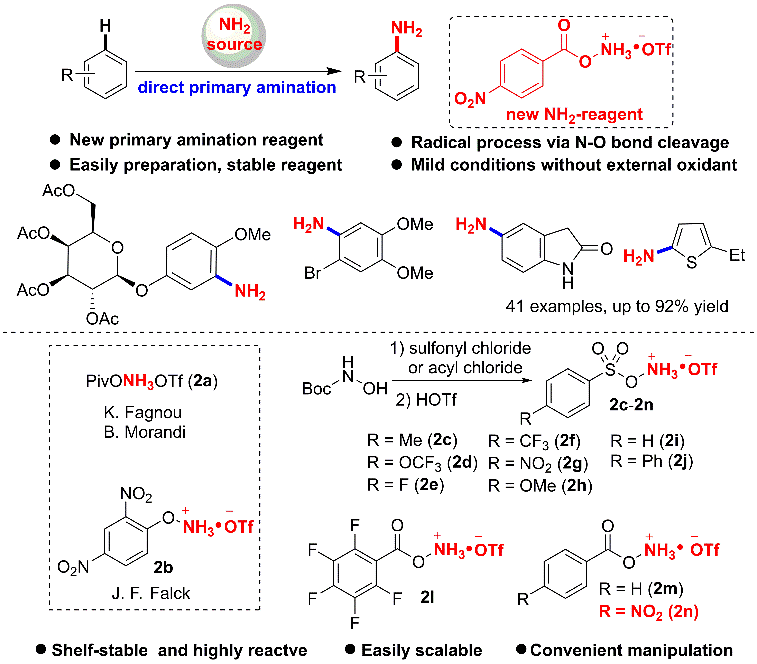
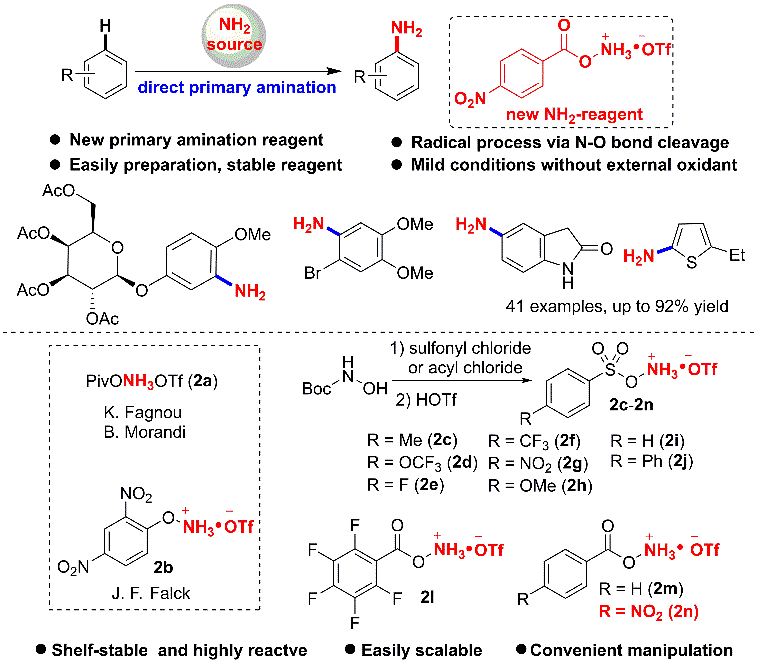
Ref.: Jianzhong Liu, Kai Wu, Tao Shen, Yujie Liang, Miancheng Zou, Yuchao Zhu, Xinwei Li, Xinyao Li, Ning Jiao*. Chem.–Eur. J. 2017, 23, 563.
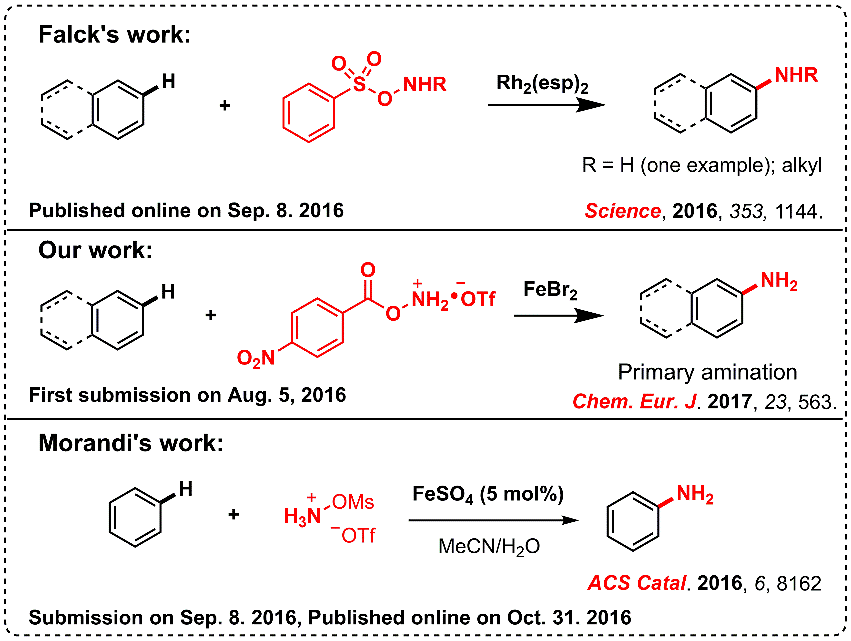
2. From alkylarenes to anilines via site-directed carbon–carbon amination.
Anilines are fundamental motifs in various chemical contexts, and are widely used in the industrial production of fine chemicals, polymers, agrochemicals and pharmaceuticals. A recent development for the synthesis of anilines uses the primary amination of C–H bonds in electron-rich arenes. However, there are limitations to this strategy: the amination of electron-deficient arenes remains a challenging task and the amination of electron-rich arenes has a limited control over region-selectivity-the formation of meta-aminated products is especially difficult. Here we report a site-directed C–C bond primary amination of simple and readily available alkylarenes or benzyl alcohols for the direct and efficient preparation of anilines, realizing a new reaction pathway for the high-quality utilization of aromatic hydrocarbons. This chemistry involves a novel C–C bond transformation and offers a versatile protocol for the synthesis of substituted anilines, which enables pharmaceutical diversification. The use of O2 as an environmentally benign oxidant is demonstrated, and studies on model compounds suggest that this method may also be used for the depolymerization of lignin. It provides an alternative advance in the development of amination chemistry and demonstrates a great potential in the academic and industrial preparation of substituted anilines.
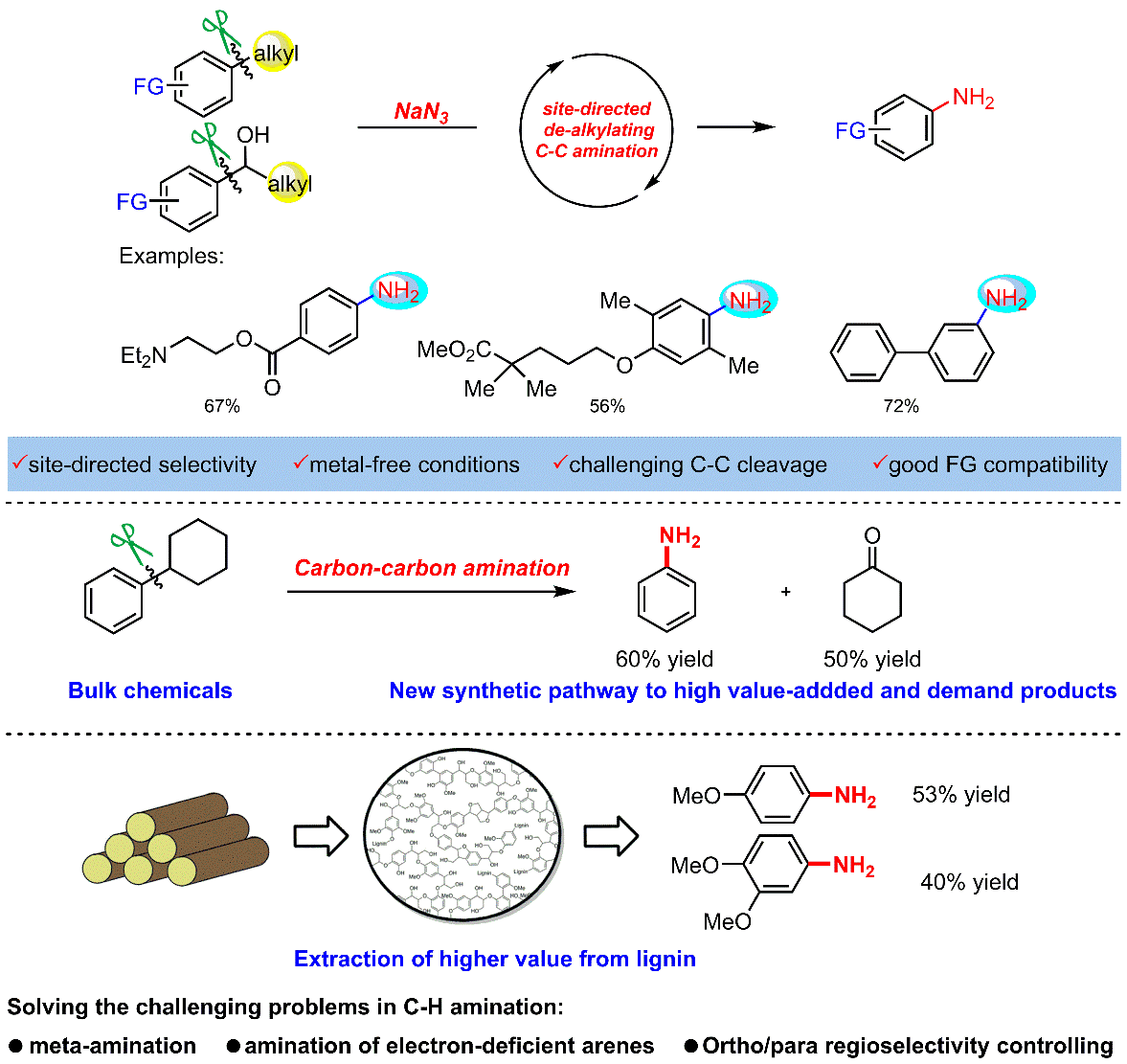
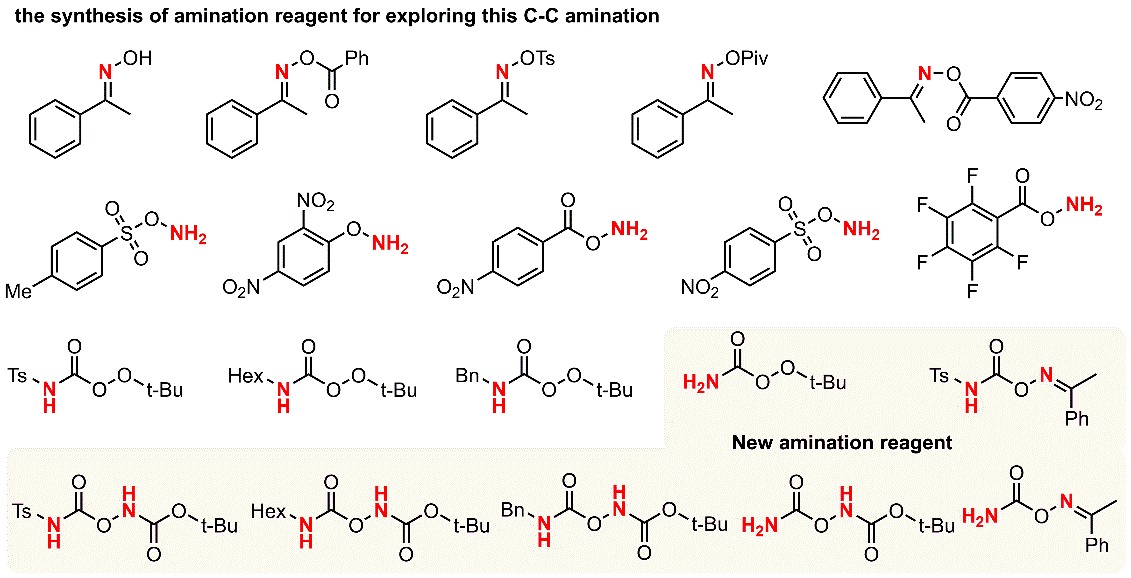
Ref.: Jianzhong Liu, Xu Qiu, Xiaoqiang Huang, Xiao Luo, Cheng Zhang, Jialiang Wei, Jun Pan, Yujie Liang, Yuchao Zhu, Qixue Qin, Song Song, Ning Jiao*. Nat Chem. 2019, 11, 71.
4. Nitromethane as a nitrogen donor in Schmidt-type formation of amides and nitriles
The Schmidt reaction has been an efficient and widely used synthetic approach to amides and nitriles since its discovery in 1923. However, its application often entails the use the of volatile, potentially explosive and highly toxic azide reagents. Here we report a sequence whereby triflic anhydride, formic and acetic acids activate the bulk chemical nitromethane to serve as a nitrogen donor in place of azides in Schmidt-like reactions. This protocol further expands the substrate scope to alkynes and simple alkyl benzenes for the preparation of amides and nitriles.
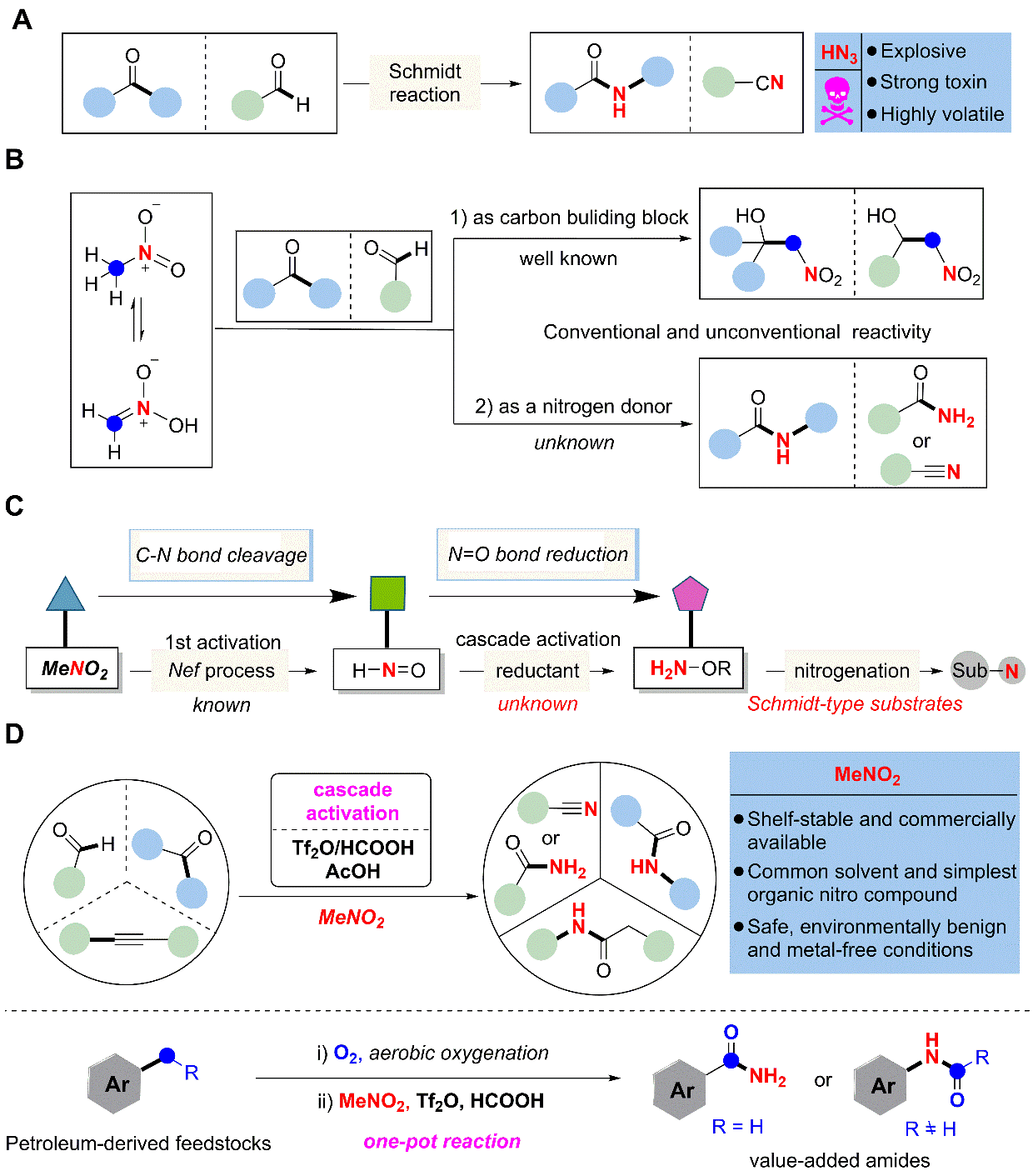
Ref.: Jianzhong Liu, Cheng Zhang, Ziyao Zhang, Xiaojin Wen, Xiaodong Dou, Jialiang Wei, Xu Qiu, Song Song, Ning Jiao* Science, 2019, 10.1126/science.aay9501.
5. Selective C(Ar)-C(alkenyl) single Bond Amination of Styrenes: Highly Efficient Approach to Primary Anilines and phenols
The abundance and diversity of styrenes (produced globally ~18.5 million tons per year) from natural sources and coal/petroleum products make them suitable buliding blocks for the assembly of synthetically valuable and structurally more complex compounds. Thus, the development of new methods and strategies for the direct transformation of styrenes is very desired and has been widely employed in organic synthesis. However, all approaches to date generally focus on the activation of activated C=C double bond, Csp2-H bond, and the allylic Csp3-H bond. Here we described a new type of activation mode of styrenes through the site-selective C(Ar)-C(alkenyl) single bond cleavage that would otherwise be more challenging to achieve, thus contributing a novel site-specific pathway for substituted anilines and phenol synthesis. The mild conditions of transition metal free, redox-neutral, wide substrates scope, simple operation enable a practical and attractive approach to anilines. To the best of our knowledges, it is the first example of the direct transformation from styrenes to anilines and phenols.
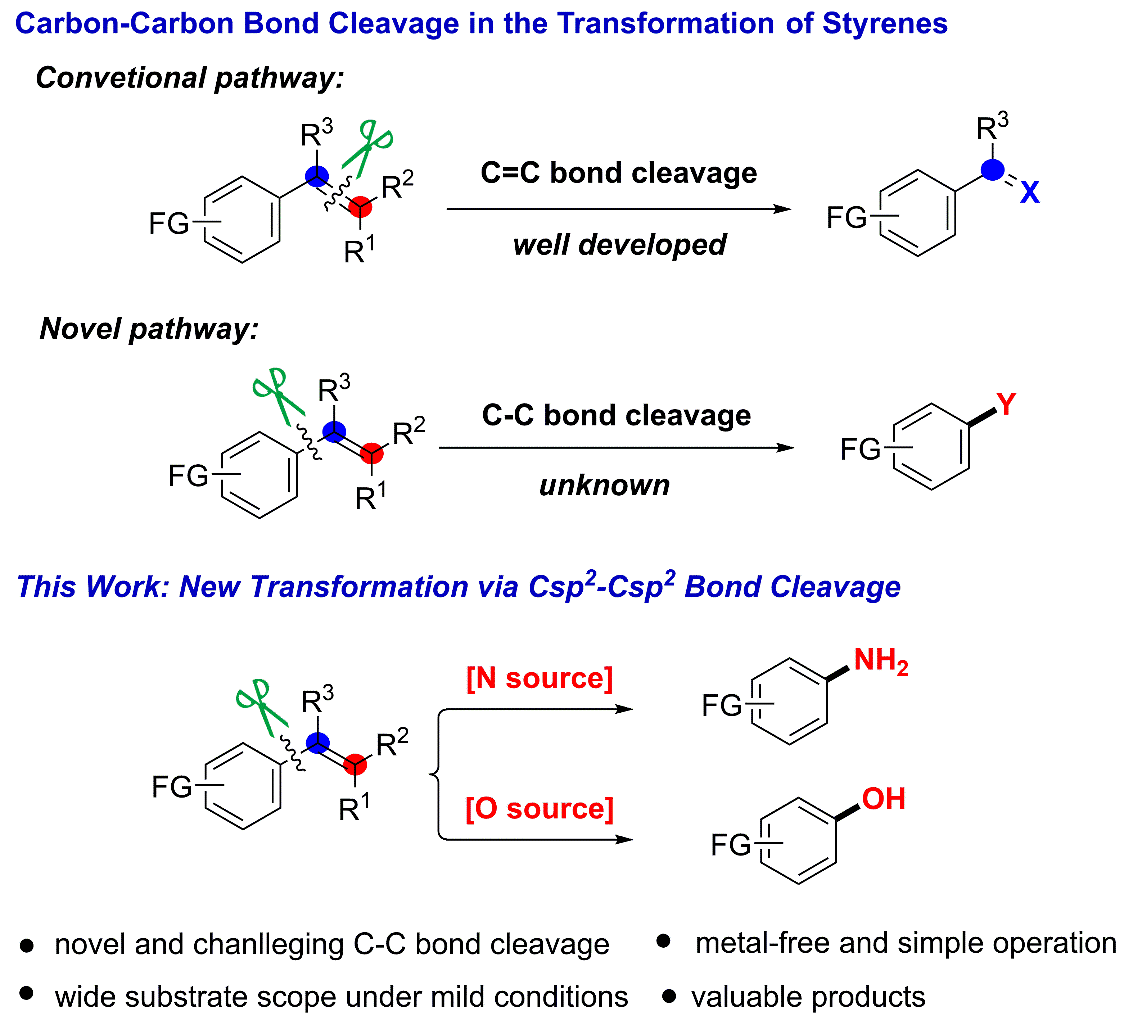
Ref.: Jianzhong Liu, Xiao Luo, Xu Qiu, Cheng Zhang, Jun Pan, Ning Jiao*. (to be submited).
4. Oxygenation of Simple Olefins through Selective Allylic C-C Bond Cleavage: A Direct Approach to Cinnamyl Aldehydes.
Selective cleavage of C-H and C–C bonds, which would provide powerful strategies to efficiently edit organic molecules, poses a great synthetic challenge. Allyl groups have acted as important skeletons in organic synthesis. In addition to considerable numbers of allylic C–H bond functionalization reactions, cleaving the corresponding allylic C-C bond is gaining increased interest and developing rapidly. A novel metal-free allylic C–C σ-bond cleavage of simple olefins to give valuable cinnamyl aldehydes is reported. 1,2-Aryl or alkyl migration through allylic C–C bond cleavage occurs in this transformation, which is assisted by an alkyl azide reagent. This method enables O-atom incorporation into simple unfunctionalized olefins to construct cinnamyl aldehydes. The reaction features simple hydrocarbon substrates, metal-free conditions, and high regio- and stereo-selectivity.

Ref.: Jianzhong Liu+, Xiaojin Wen+, Chong Qin+, Xinyao Li, Xiao Luo, Ao Sun, Bencong Zhu, Song Song*, Ning Jiao*. Angew. Chem. Int. Ed. 2017, 56, 11940.
6. Pd(II)-Catalyzed Pyridine N-Oxides Directed Arylation of Unactivated Csp3-H bonds.
Heteroatom directed transition-metal-catalyzed C-H functionalization
provides a concise access to the site-selectively
constructing carbon-carbon bonds. In the past decade, remarkable progress in this field has been achieved based on C(sp2)‒H bond activation. In comparison, transition metal-catalyzed unactivated
Csp3-H functionalization is quite difficult because of the entropic
factors and the possible b-H elimination from the metalated alkyl intermediates. Mono-dentate and bidentate auxiliary-directed group could efficiently enhance the unactivated Csp3-H
bond. Here we developed novel bidentate directing groups for exploring the
potential novel C-H functionalization reactions.
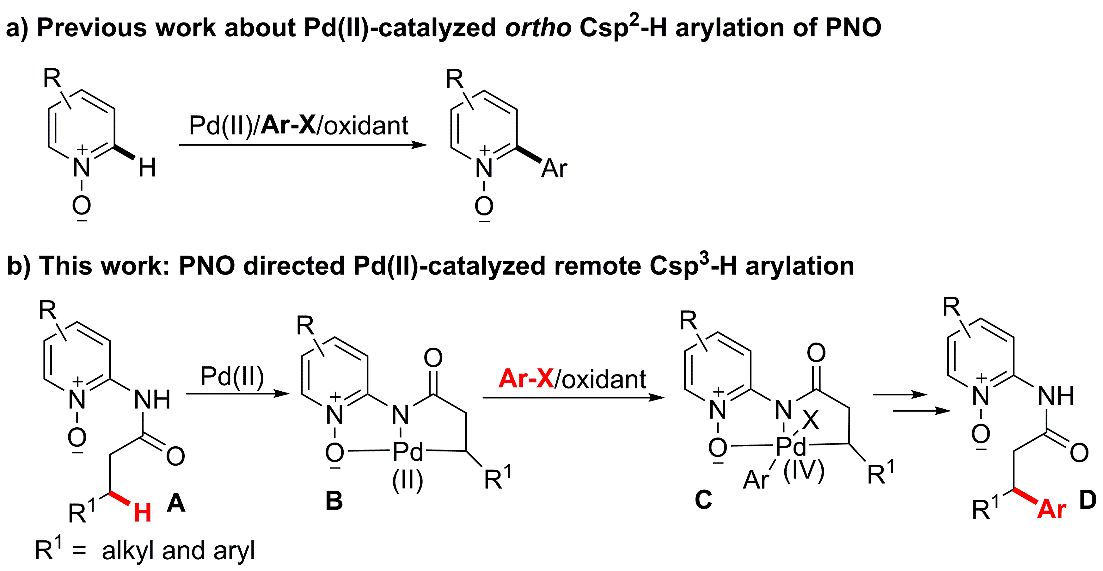
Ref.: Jianzhong Liu, Ying Xie, Wei Zeng*, Dongen Lin*, Yuanfu Deng*, Xiaoxia Lu*. J. Org. Chem. 2015, 80, 4618.
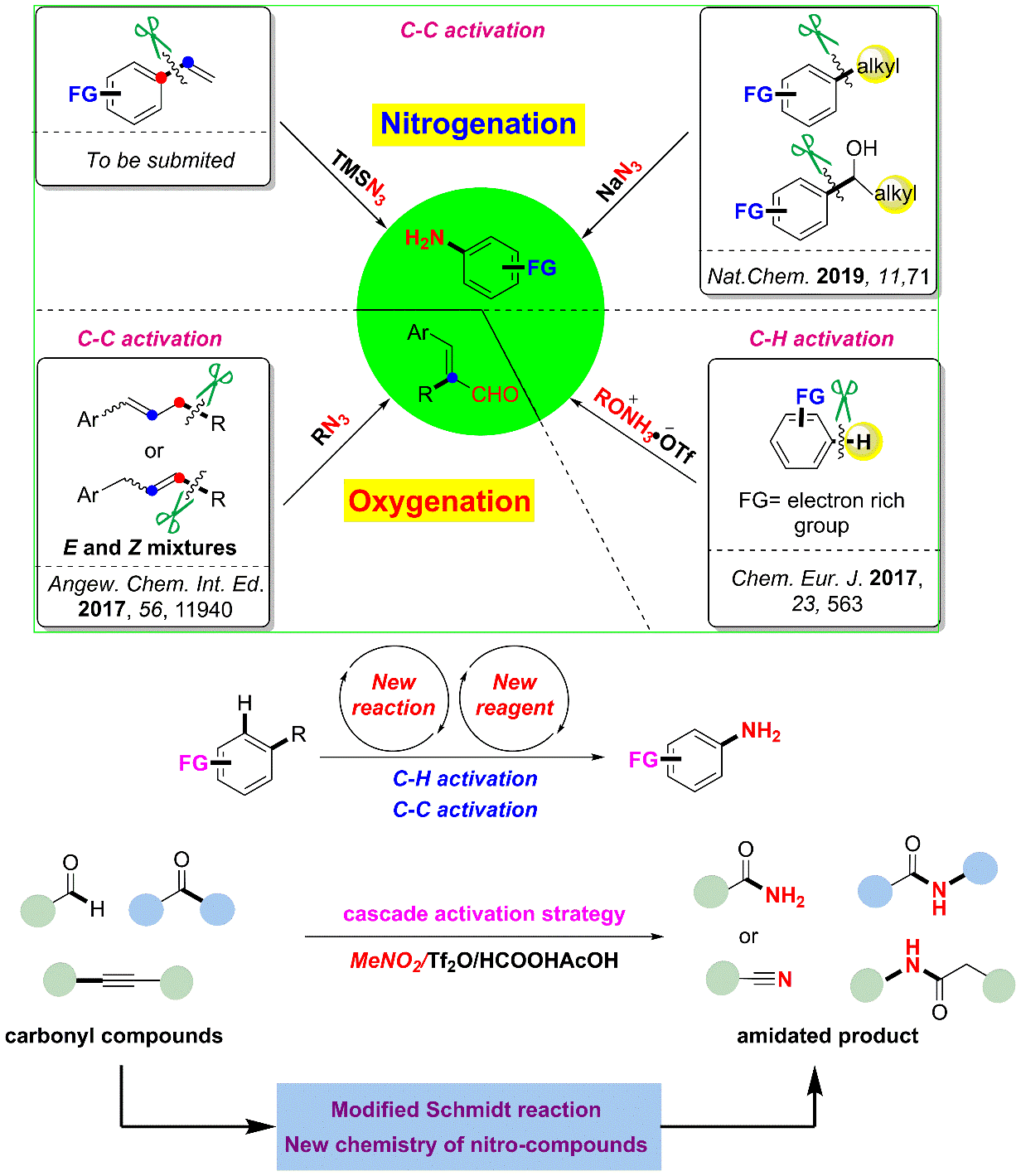
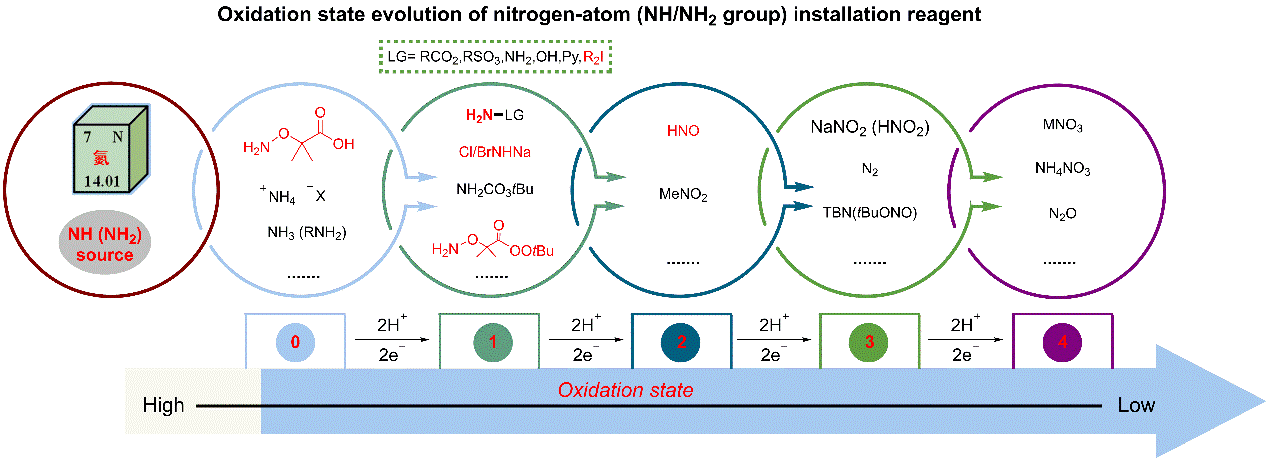
Research Summary of
My PhD’ Time (two works in preparation).
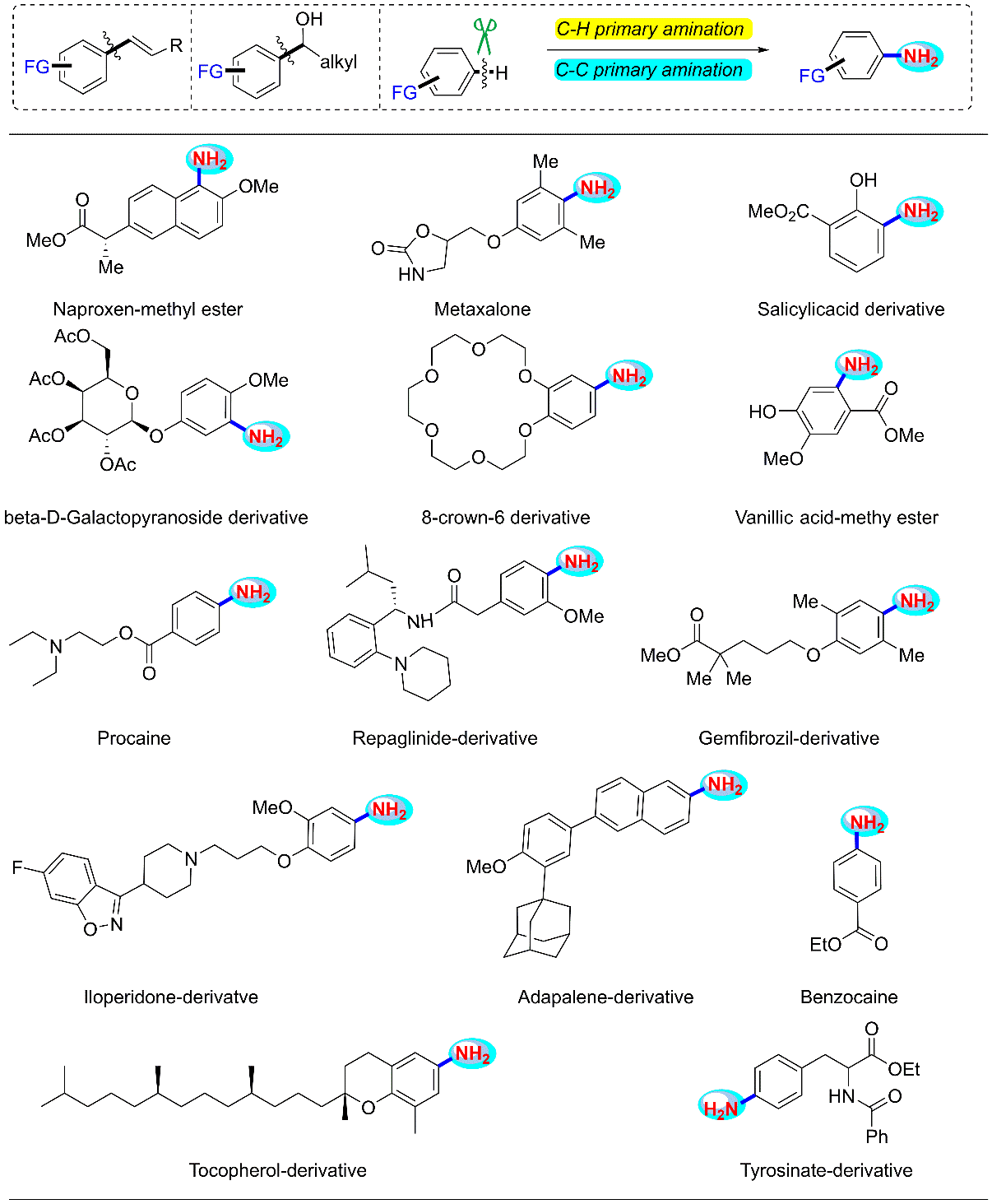
The efficient synthesis
or modification of natural products and drugs (Part 1).
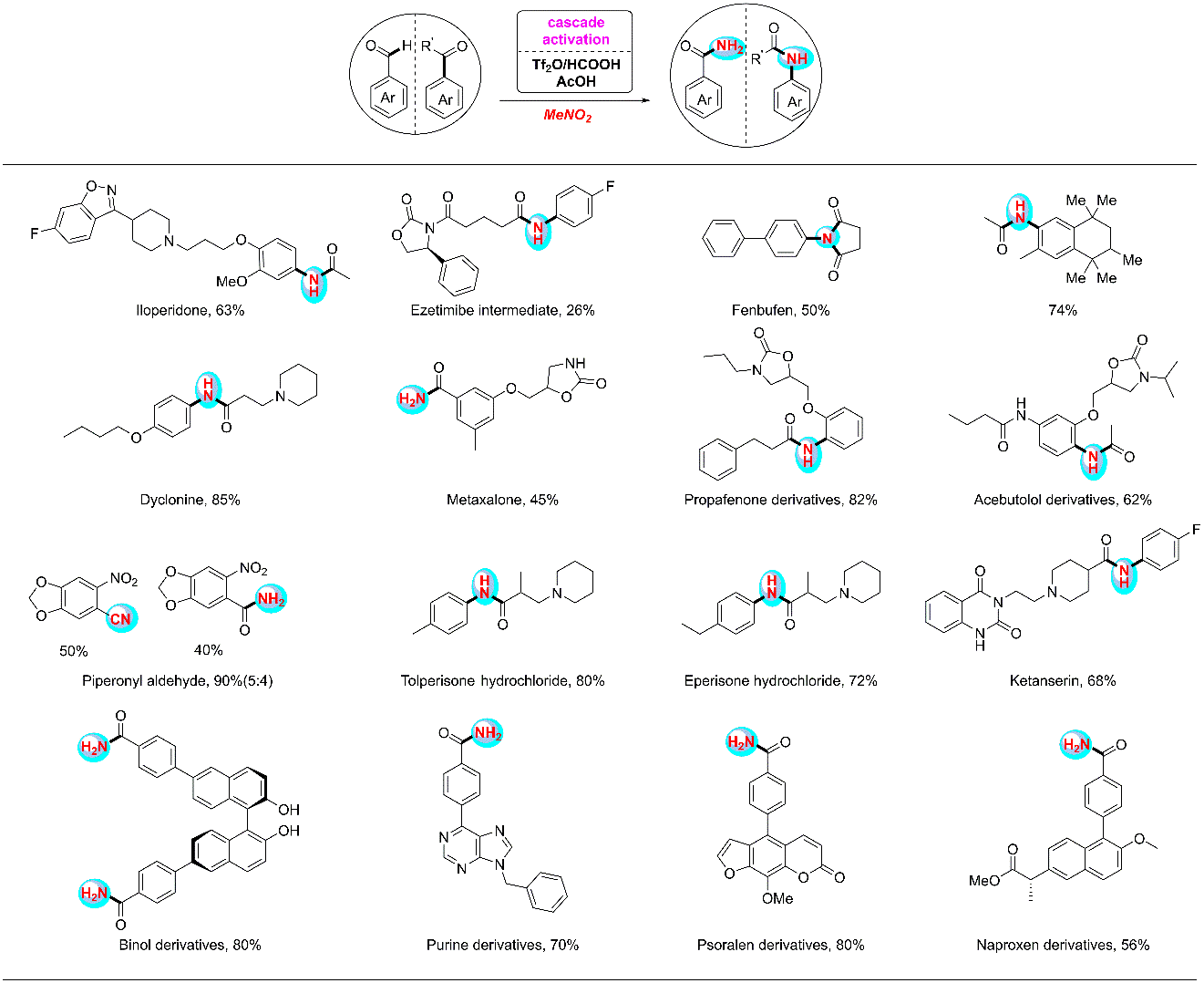
The efficient synthesis or modification of natural products and drugs (Part 2).